Breast Health Studies
-
- The evolving role of the dynamic thermal analysis in the early detection of breast cancer
- Evaluation of digital infra-red thermal imaging as an adjunctive screening method for breast carcinoma: A pilot study.
- Effectiveness of a non-invasive digital infrared thermal imaging system in the detection of breast cancer.
- Thermal detection of embedded tumors using infrared imaging.
- Beating Breast Cancer.
- Medical Devices and Systems.
- Circadian rhythm chaos: a new breast cancer marker.
- Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer.
- Medical Infrared Imaging of the Breast: An Analysis of 100 Successive Cases of Breast Cancer.
The evolving role of the dynamic thermal analysis in the early detection of breast cancer
M Salhab, W Al Sarakbi and K Mokbel*ABSTRACT
It is now recognized that the breast exhibits a circadian rhythm which reflects its physiology. There is increasing evidence that rhythms associated with malignant cells proliferation are largely non-circadian and that a circadian to ultradian shift may be a general correlation to neoplasia. Cancer development appears to generate its own thermal signatures and the complexity of these signatures may be a reflection of its degree of development. The limitations of mammography as a screening modality especially in young women with dense breasts necessitated the development of novel and more effective screening strategies with a high sensitivity and specificity. Breast thermography or dynamic thermal analysis of the breast is a safe, non invasive approach that seems to be sensitive for the early detection of breast cancer. This article focuses on dynamic thermal analysis as an evolving method in breast cancer detection in pre-menopausal women with dense breast tissue. Prospective multi-center trials are required to validate this promising modality in screening. The issue of false positives require further investigation using molecular genetic markers of malignancy and novel techniques such as mammary ductoscopy.INTRODUCTION
Breast cancer is one of the most common cancers, it is estimated that one in eight women in the USA will develop breast cancer during their lifetime. Furthermore, 25–30% of breast cancers are found in pre-menopausal women [1]. Currently mammography is the best available approach for the early detection of breast cancer in the general population with a sensitivity of 75–90%. However, the positive predictive value is only 25%. In addition to mammography, non invasive new modalities have been developed to allow the early detection of breast cancer in all age groups and more importantly in young women with dense breast tissue and women who have high risk of developing breast cancer such as, women with strong family history and carriers of BRCA1 and/or BRCA2 genes. Currently, magnetic resonance imaging (MRI) is being studied for the early detection of breast cancer. Its sensitivity in high risk women has been found to be much higher than mammography but with a lower specificity. Kriege et al observed a higher sensitivity for MRI in detection of breast cancer in women with a genetic predisposition or at high risk compared to (71% vs. 41 %) but with lower specificity (90% vs. 95%). Electrical impedance scanning (EIS) is another modality under development for breast cancer detection especially in young women with dense breasts. The basic science behind its use is the fact that malignant tumors have lower electrical impedance than the surrounding normal tissue. However, separation between malignant and benign lesions needs further investigations. Furthermore, mammary ductoscopy (MD) and visualization of mammary ducts and proteomics of nipple aspirate fluid (NAF) and serum are promising screening modalities that require further evaluation. The limitations of mammography as a screening modality especially in young women with dense breasts necessitated the development of novel and more effective screening strategies with a high sensitivity and specificity. This article focuses on the dynamic thermal analysis as an evolving non invasive and a safe method in breast cancer detection in pre-menopausal women with dense breast tissue and women at high risk due to family history or genetic predisposition.BREAST AND CIRCADIAN RHYTHM
It is now recognized that the establishment and growth of a tumor depend on neovascularization. This successful recruitment of new blood vessels into a tumor; also known as angiogenesis is dependent on angiogenic growth factors produced by the tumor cells. Such new vessels grow adjacent to the tumor presumably to increase its nutrient supply. These new vessels lack smooth muscles rendering them unreceptive to control by epinephrine. The lack of receptivity produce a more constant blood flow, thus increasing the local temperature. Earlier technology for assessing thermal abnormalities in the breast focused on the presence of the abnormal temperature as a crucial marker. In a study conducted by Gantherine et al, 21.3% of patients who had abnormal thermograms but no abnormality on physical examination and mammography developed breast cancer within the next 3 years. In another study of women who had thermal abnormalities on initial examination using infrared technology, long term follow up (2–10 years) revealed that 33% of these women developed breast cancer, a rate six times higher than that expected in the normal population. This relationship between breast skin temperature and breast cancer was thoroughly examined by Gros et al. They found that the differences between the characteristics of rhythmic changes in skin temperature of clinically healthy and cancerous breasts were real and measurable. Despite these interesting observations thermography as a general screening tool for the detection of women at risk of breast cancer did not find a wide spread acceptance due to low sensitivity of the test and the subjective nature of the test interpretations. The superficial thermal patterns measured on the surface of the breast seem to be related to tissue metabolism and vascularization within the underlying tissue. Such thermal patterns change significantly as a result of normal phenomena including menstrual cycle, pregnancy and more importantly the pathologic process itself. Additionally, cancer development represents the summation of a large number of mutations that occur over years, each with its own particular histologic phenotype. Such changes appear to generate their own thermal signature and the complexity of these signatures may be a reflection of their degree of development. Temperature in a normal breast increases from the skin into the deep tissue and heat conductivity in the healthy breasts is constant in most cases and generally can be characterized in terms of circadian rhythm periodicity. In contrast, the rhythms associated with malignant cells proliferation are largely non circadian and suggest that a circadian to ultradian shift may be a general correlation to neoplasia. Heat production by the tumor under the influence of angiogenesis should be therefore re-examined in terms of absence of normal circadian fluctuations. Due to the increased blood flow and the lack of receptivity in the newly formed vessels in malignancy, temperature production exhibits circadian rhythmic variations to a far lesser degree than is evident in the healthy breasts. It has been found that independent of a tumor’s size, relatively small tumors (>/= 0.5 cm in diameter), poorly vascularized rapidly growing tumors can produce increases in regional heat. The explanation for this effect is unclear but it may be due to the chronic inflammatory response around developing breast tumors. With increasing evidence that inflammation can enhance tumor growth and is associated with a poor prognosis, this suggestion implies that thermal analysis may have considerable value. Furthermore, the unique relationship between the thermal circadian rhythm and mitotic activity could be considered as a first warning of tumor development, which can be detected using a safe and non-invasive technology. The genes that drive the circadian rhythm are emerging as central players in gene regulation throughout the organism, particularly for cell-cycle regulatory genes and the genes of apoptosis.DYNAMIC THERMAL ANALYSIS
Recent technological advances have facilitated the recording of circadian rhythm variations of the breast and analyzing the recorded data using highly complicated computer statistical software. A miniaturized microprocessor has been developed to record and store thermal information collected from eight separate sites of each breast. Sensors are placed in anatomically critical positions elicited by data obtained from tumor registries as to where cancers are most likely to develop. In the First Warning System (FWS, Lifeline Biotechnologies, Florida, USA), thermal data are collected every five minutes for a period of 48 hours during which time women are encouraged to maintain their daily activities. 9000 pieces of data are recorded by microprocessors during the test period and analyzed using specially developed statistical software. Temperature points from each contralateral sensor are plotted against each other to form a thermal motion picture of a lesion’s physiological activity. Such a technology was first used by Farrar et al who examined a cohort of 138 women who had been scheduled for open breast biopsies based on the finding of physical examination and mammography. A total of 23 women (17%) were found to have breast cancer, of these, 20 (87%) were characterized by the monitor as being high risk. The other 3 patients (13%) who were missed by the monitor had ductal carcinoma. Mammography was positive or suspicious in only 19 patients (83%). Of the 4 cancers missed by mammography (3 of them were pre menopausal), the monitor correctly characterized 3 women as being high risk. A neural net algorithm was subsequently developed and evaluated by the authors because of its value in analyzing the non-linear data such as these recorded by the breast’s monitors. Using this neural net algorithm reduced the number of false positives (18% vs. 30%)) and improved sensitivity (91% vs. 87%). One of the main challenges to this technology is the false positive cases; confusion could be created in these women who are characterized as being positive or high risk by dynamic thermal analysis in the absence of physical and mammographical signs. This group of women may or may not have cancer in its earliest stages. Further retrospective analysis of the thermal data using a refined neural net algorithm may increase the sensitivity and reduce the number of false positives. Also this group of patients may well benefit from the new advances in the nipple aspirate fluid analysis and proteomic profiling technologies. Research is currently ongoing on this subject and the initial results are promising.THE FUTURE
Dynamic thermal analysis of the breast is a safe, non invasive approach that seems to be sensitive for the early detection of breast cancer especially in young women where the conventional mammography is of limited value. Such a technology could become the initial breast screening test in pre-menopausal women and those who are classified as positive can then be selected for anatomical imaging with mammography, MRI and/or ultrasonography. Further refinement of the neural net algorithm is required in order to shorten the period of data recording and improve specificity. Prospective multi-centre trials are then required to validate these promising observations. The issue of false positives require further investigation using molecular genetic markers of malignancy and novel techniques such as mammary ductoscopy. Finally, a better understanding of the circadian rhythm biology and clearer definition of the thermal activity boundaries for various pathological conditions of the breast will open the door to a new and more precise screening method for breast cancer.REFERENCES
1. Keith LG, Oleszczuk JJ, Laguens M: Circadian rhythm chaos: A new breast cancer marker. Int J Fertil Womens Med 2001, 46(5):238-247. 2. Donegan WL: Evaluation of a palpable breast mass. N Engl J Med 1992, 327:937-942. 3. Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW:Ten- year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med 1998, 338:1089-1096. 4. Harris JR, Lippman ME, Veronesi U, Willet W: Breast cancer (1). N Engl J Med 1992, 327:317-328. 5. Elmore JG, Armstrong K, Lehman CD, Fletcher SW: Screening for breast cancer. JAMA 2005, 293(10):1245-1256. 6. Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linhorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers T, Klijn JG, the Magnetic Resonance Imaging Screening Study Group: Efficacy of Magnetic Resonance Imaging and Mammography for Breast Cancer Screening in Women With a Familial or Genetic Predisposition. Obstet Gynecol Surv 2005, 60(2):107-109. 7. Hope TA, Iles SE: Technology review: the use of electrical impedance scanning in the detection of breast cancer. Breast Cancer Res 2004, 6(2):69-74. Epub 2003 Nov 13. 8. Zou Y, Guo Z: A review of electrical impedance techniques for breast cancer detection. Med Eng Phys 2003, 25(2):79-90. 9. Pawlik TM, Fritsche H, Coombes KR, Xiao L, Krishnamurthy S, Hunt KK, Pusztai L, Chen JN, Clarke CH, Arun B, Hung MC, Kuerer HM:Significant differences in nipple aspirate fluid protein expression between healthy women and those with breast cancer demonstrated by time-of-flight mass spectrometry. Breast Cancer Res Treat 2005, 89(2):149-157. 10. Mokbel K, Escobar PF, Matsunaga T: Mammary ductoscopy: current status and future prospects. Eur J Surg Oncol 2005, 31(1):3-8. 11. La Vecchia C, Parazzini F, Franceshi S, Decarli A: Risk factors for benign breast disease and their relation with breast cancer risk. Pooled information from epidemiologic studies. Tumori 1985, 71:167-178. 12. Folkman J: Introduction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989, 339:58-61. 13. Mc Donald D: Mechanism of Tumour Leakiness proceeding angiogenesis and cancer. From basic mechanisms to therapeutic applications. In American association of cancer research conference Traverse City, Michigan. 2000 October 11–15. 14. Farrar WB, Patricia R, Sexton RN, Marsh W, Olsen J: An evaluation of a new objective method for breast cancer screening. In Scientific exhibit presented at the 76th Annual clinical congress of the American college of surgeons San Francisco, California. October 8–11, 1990. 15. Gros C, Gautherine M, Bourjat P: Prognosis and post therapeutic followup of breast cancers by thermography Edited by: Aarts NJM, Gautherine M, Ring EFJ. Thermography. Karger, Basel; 1975:77-90. 16. Gautherine M, Gros C: Contribution of infrared thermography to early diagnosis, pretheraputic prognosis and post-irradiation follow-up of breast carcinomas. Med Mundi 1976, 21:135-149. 17. Gautherine M, Haehnel P, Walter JP, Keith LG: Thermovascular changes associated within situ and minimal breast cancers; results of an ongoing prospective study after four years 1987, 11:833-842. 18. Feig SA: Role and evaluation of mammography and other imaging methods for breast cancer detection, diagnosis, and staging. Semin Nucl Med 1999, 29(1):3-15. 19. Simpson HW, Mutch F, Halberg F, Griffiths K, Wilson D: Bimodal age-frequency of epitheliosis in cancer mastectomies. Cancer 1982, 50:2417-2422. 20. Simpson HW, Griffiths K: The diagnosis of pre-cancer by the chronobra. I: Background review. Chronobiol Int 1989, 6:355-369. 21. Echave Llanos HM, Nash RE: Mitotic circadian rhythms in hepatoma. J Nat Cancer Inst 1970, 44:581-585. 22. Garcia-Sainz M, Halberg F: Mitotic rhythm in human cancer, reevaluated by electronic computer programs: Evidence of temporal pathology. J Nat Cancer Inst 1966, 37:279-292. 23. Nash RE, Echave Llanos HM: 24-hour variations in DNA-synthesis of a fast growing and slow growing hepatoma: DNA synthesis rhythm in hepatoma. 1971, 47:1007-1012. 24. Gautherine M: thermobiological assessment of benign and malignant breast disease. Am J Obstet Gynecol 1983, 147:461. 25. Stefanadis C, Chrysohoou C, Paraskevvas E, Panagiotakos DB, Xynopoulos D, Dimitroullopolulos D, et al.: Thermal heterogeneity constitutes a marker for detection of malignant gastric lesion in vivo. J Clin Gastroenterol 2003, 36(3):215-218. 26. Stefanadis C, Chrysohoou C, Paraskevvas E, Panagiotakos DB, Xynopoulos D, Dimitroullopolulos D, et al.: Increased temperature of malignant urinary bladder tumours in vivo: The application of a new method based on a catheter technique. J Clin Gastroenterol 2001, 1(3):676-681. 27. Stefanadis C, Chrysohoou C, Panagiotakos DB, Passalidou E, Kasti V, Polychronopoulos V, Toutouzas : Temperature differences are associated with malignancy on lung lesions : A clinical study. BMC Cancer 2003, 3:1. Epub 28. Head JF, Wang F, Eilliott RL: Breast thermography is a non-invasive prognostic procedure that predicts tumour growth rate in breast cancer patients. Ann NY Acad Sci 1993, 698:153-158. 29. Gautherine M: Thermopathology of breast cancer: Measurement and analysis of in vivo temperature and blood flow. In:Thermal characteristics of Tumours: Application in detection and treatment. Ann NY Acad Sci 1980, 335:383-415. 30. Xie W, McCahon P, Jakobsen K, Parish C: Evaluation of the ability of digital infrared imaging to detect vascular changes in experimental animal tumors. Int J Cancer 2004, 108(5):790-4. 31. Stevens RG: Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology 2005, 16(2):254-8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1084358/Evaluation of digital infra-red thermal imaging as an adjunctive screening method for breast carcinoma: A pilot study.
Rassiwala M1, Mathur P2, Mathur R3, Farid K3, Shukla S3, Gupta PK4, Jain B4.ABSTRACT
BACKGROUND
Early screening plays a pivotal role in management of breast cancer. Given the socio-economic situation in India, there is a strong felt need for a screening tool which reaches the masses rather than waiting for the masses to reach tertiary centers to be screened. Digital infrared thermal imaging (DITI) or breast thermography as a screening test offers this possibility and needs to be carefully assessed in Indian scenario.METHODS
The study involved 1008 female patients of age 20-60 years that had not been diagnosed of cancer of breast earlier. All the subjects in this population were screened for both the breasts using DITI. Based on the measured temperature gradients (ΔT) in thermograms, the subjects were classified in one of the three groups, normal (ΔT ≤ 2.5), abnormal (ΔT > 2.5, <3) and potentially having breast cancer (ΔT ≥ 3). All those having (ΔT > 2.5) underwent triple assessment that consisted of clinical examination, radiological and histopathological examination. Those with normal thermograms were subjected to only clinical examination.RESULTS
Forty-nine female breasts had thermograms with temperature gradients exceeding 2.5 and were subjected to triple assessment. Forty one of these which had ΔT ≥ 3 were proven to be having cancer of breast and were offered suitable treatment. Eight thermograms had temperature gradients exceeding 2.5 but less than 3. Most of these were lactating mothers or had fibrocystic breast diseases. As a screening modality, DITI showed sensitivity of 97.6%, specificity of 99.17%, positive predictive value 83.67% and negative predictive value 99.89%.CONCLUSION
Based on the results of this study involving 1008 subjects for screening of breast cancer, breast thermography turns out to be a very useful tool for screening. Because it is non-contact, pain-free, radiation free and comparatively portable it can be used in as a proactive technique for detection of breast carcinoma. International Seminars in Surgical Oncology http://www.ncbi.nlm.nih.gov/pubmed/25448668Effectiveness of a non-invasive digital infrared thermal imaging system in the detection of breast cancer.
Arora N, Martins D, Ruggerio D, Tousimis E, Swistel AJ, Osborne MP, Simmons RM Department of Surgery, New York Presbyterian Hospital-Cornell, New York, NY, USA.BACKGROUND
Digital infrared thermal imaging (DITI) has resurfaced in this era of modernized computer technology. Its role in the detection of breast cancer is evaluated.METHODS
In this prospective clinical trial, 92 patients for whom a breast biopsy was recommended based on prior mammogram or ultrasound underwent DITI. Three scores were generated: an overall risk score in the screening mode, a clinical score based on patient information, and a third assessment by artificial neural network.RESULTS
Sixty of 94 biopsies were malignant and 34 were benign. DITI identified 58 of 60 malignancies, with 97% sensitivity, 44% specificity, and 82% negative predictive value depending on the mode used. Compared to an overall risk score of 0, a score of 3 or greater was significantly more likely to be associated with malignancy (30% vs 90%, P < .03).CONCLUSION
DITI is a valuable adjunct to mammography and ultrasound, especially in women with dense breast parenchyma. http://www.ncbi.nlm.nih.gov/pubmed/18809055Thermal detection of embedded tumors using infrared imaging.
Mital M, Scott EP. Department of Mechanical Engineering, Virginia Tech, Blacksburg, VA 24060, USA. Breast cancer is the most common cancer among women. Breast thermography, also known as thermal imaging or infrared imaging, is a procedure to determine if an abnormality is present in the breast tissue temperature distribution. This abnormality in temperature distribution might indicate the presence of an embedded tumor. Although thermography is currently used to indicate the presence of an abnormality, there are no standard procedures to interpret these and determine the location of an embedded tumor. This research is a first step towards this direction. It explores the relationship between the characteristics (location and power) of an embedded heat source and the resulting temperature distribution on the surface. Experiments were conducted using a resistance heater that was embedded in agar in order to simulate the heat produced by a tumor in the biological tissue. The resulting temperature distribution on the surface was imaged using an infrared camera. In order to estimate the location and heat generation rate of the source from these temperature distributions, a genetic algorithm was used as the estimation method. The genetic algorithm utilizes a finite difference scheme for the direct solution of the Pennes bioheat equation. It was determined that a genetic algorithm based approach is well suited for the estimation problem since both the depth and the heat generation rate of the heat source were accurately predicted. https://www.ncbi.nlm.nih.gov/pubmed/17227096Beating Breast Cancer
William Hobbins, MD, FABS, DABCT, FIACT William Amalu, DC, DABCT, DIACT, FIACT
 This year, over 192,000 women will be diagnosed with breast cancer in the US and 1.2 million worldwide (Source: American Cancer Society and WHO). As shocking as these numbers are, even worse is the number of cancers that won’t be detected until it’s too late. The consensus among experts is that early detection holds the key to survival. Although this is true, detection is not occurring early enough. Even though women are advised to begin having mammograms at 40, what they don’t know is that by the time most cancers are detected they have been growing for 10 years, and that 20% of all cancers can’t be seen by a mammogram. It is because of these factors, and others, that the number of women who die from this disease has gone relatively unchanged in the past 40 years.
This year, over 192,000 women will be diagnosed with breast cancer in the US and 1.2 million worldwide (Source: American Cancer Society and WHO). As shocking as these numbers are, even worse is the number of cancers that won’t be detected until it’s too late. The consensus among experts is that early detection holds the key to survival. Although this is true, detection is not occurring early enough. Even though women are advised to begin having mammograms at 40, what they don’t know is that by the time most cancers are detected they have been growing for 10 years, and that 20% of all cancers can’t be seen by a mammogram. It is because of these factors, and others, that the number of women who die from this disease has gone relatively unchanged in the past 40 years.
If a significant change in breast cancer mortality is to be realized, we have to rethink how we are providing for early detection. Are we currently providing a system that includes an early warning? What if we had a system that would comprise a multi-modality approach that includes technologies that reflect the early cancerous process itself? If there were a method of very early detection, a procedure that may act as an early warning system, women would have an additional tool to give them the fighting chance they need to win this battle. What is needed is a risk marker. We may be able to turn these statistics around if a risk marker were added to a woman’s regular screening procedures. Women now have access to a unique technology that may give them this early risk marker; a procedure called Digital Infrared Imaging (DII)
how we are providing for early detection. Are we currently providing a system that includes an early warning? What if we had a system that would comprise a multi-modality approach that includes technologies that reflect the early cancerous process itself? If there were a method of very early detection, a procedure that may act as an early warning system, women would have an additional tool to give them the fighting chance they need to win this battle. What is needed is a risk marker. We may be able to turn these statistics around if a risk marker were added to a woman’s regular screening procedures. Women now have access to a unique technology that may give them this early risk marker; a procedure called Digital Infrared Imaging (DII)
DII is a technology that uses advanced high-resolution computerized medical infrared camera  systems to detect and analyze thermovascular heat patterns from the surface of the breasts. When a cancer is forming it incorporates and develops its own blood supply in order to feed its growth (a process known as angiogenesis). Even more important, pre-cancerous tissues may start this process in advance of the cells becoming malignant. This increased blood supply causes an abnormal heat pattern in the breasts. DII can detect this abnormal heat pattern by using specialized infrared cameras and sophisticated computerized analysis under the guidance of a doctor who is board certified in the procedure. These abnormal heat patterns are among the earliest known signs of risk that a cancer may be a forming.
systems to detect and analyze thermovascular heat patterns from the surface of the breasts. When a cancer is forming it incorporates and develops its own blood supply in order to feed its growth (a process known as angiogenesis). Even more important, pre-cancerous tissues may start this process in advance of the cells becoming malignant. This increased blood supply causes an abnormal heat pattern in the breasts. DII can detect this abnormal heat pattern by using specialized infrared cameras and sophisticated computerized analysis under the guidance of a doctor who is board certified in the procedure. These abnormal heat patterns are among the earliest known signs of risk that a cancer may be a forming.
An increased level of early detection may be realized when DII is added to a woman’s regular breast health care. It has been found that an abnormal thermal image is the single most important sign of high risk for developing breast cancer, 10 times more significant than a first order family history of the disease. This gives DII the ability to act as a possible risk marker; thus, warning a woman about her own unique level of future risk for breast cancer.
W omen who undergo the test find it to be fairly uneventful, since the procedure uses no radiation or contact with the breasts.
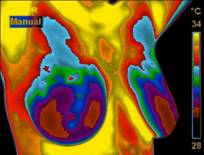
omen who undergo the test find it to be fairly uneventful, since the procedure uses no radiation or contact with the breasts.  Women with dense breasts, implants, and women who are pregnant or nursing can be imaged without any harm or reduction in the accuracy of the test. Normal images, like the one seen on the left, show evenly cool inactive breasts (dark colors represent cold areas). Abnormal images, as seen on the right, show highly active blood vessels giving off heat in one breast. Since the procedure does not pose any harm to the patient, women who are at higher risk can be monitored closely without adverse effects on their health.
Women with dense breasts, implants, and women who are pregnant or nursing can be imaged without any harm or reduction in the accuracy of the test. Normal images, like the one seen on the left, show evenly cool inactive breasts (dark colors represent cold areas). Abnormal images, as seen on the right, show highly active blood vessels giving off heat in one breast. Since the procedure does not pose any harm to the patient, women who are at higher risk can be monitored closely without adverse effects on their health.
 Another benefit of this technology is its possible role in prevention. Digital Infrared Imaging has the added ability to observe specific thermal signs that may indicate hormonal effects on the breasts. At this time, research has determined that the single greatest risk factor for the future development of breast cancer is lifetime exposure of the breasts to estrogen. In which case, controlling the influence of estrogen on the breasts may be the single greatest method of breast cancer prevention. When hormone activity in the breast is dominated by estrogen, a specific type of infrared image is produced; thus, warning the patient and her doctor that this condition may exist. With this information in hand, a woman’s doctor will run further tests to confirm the condition and its cause. Once this is identified, a woman and her doctor can take a pro-active role in prevention. A treatment program aimed at restoring the normal hormonal balance in the breasts would follow and be monitored by the patient’s doctor. Once the hormone balance has been restored to the breasts, a woman’s overall breast cancer risk may be greatly reduced.
Another benefit of this technology is its possible role in prevention. Digital Infrared Imaging has the added ability to observe specific thermal signs that may indicate hormonal effects on the breasts. At this time, research has determined that the single greatest risk factor for the future development of breast cancer is lifetime exposure of the breasts to estrogen. In which case, controlling the influence of estrogen on the breasts may be the single greatest method of breast cancer prevention. When hormone activity in the breast is dominated by estrogen, a specific type of infrared image is produced; thus, warning the patient and her doctor that this condition may exist. With this information in hand, a woman’s doctor will run further tests to confirm the condition and its cause. Once this is identified, a woman and her doctor can take a pro-active role in prevention. A treatment program aimed at restoring the normal hormonal balance in the breasts would follow and be monitored by the patient’s doctor. Once the hormone balance has been restored to the breasts, a woman’s overall breast cancer risk may be greatly reduced.
With the incidence of breast cancer rising in women under 40, an effort to provide some form of additional test is needed in this age group. Very early detection is especially important since breast cancers in younger women are commonly more aggressive resulting in lower survival rates. Current screening procedures have proven to be inaccurate in women in this age group due to breast tissue density and other factors. These issues, however, do not affect Digital Infrared Imaging. With this technology, women under 40 now have a safe imaging procedure that they can add to their regular breast health check ups.
Digital Infrared Imaging is a high-tech non-invasive imaging procedure designed to be used by women of all ages. The technology has been thoroughly researched for over 30 years and is FDA approved for use as an adjunctive imaging tool. Its unique ability to play a possible role in prevention is an impressive added benefit. Unfortunately, at this time there are too few qualified DII centers worldwide. However, with an increasing demand for the technology, educational organizations such as the International Academy of Clinical Thermology, International Thermographic Society, and the American Academy of Thermology are providing training for certified technicians and thermologists. It is their goal to provide women with greater access to this lifesaving technology.
Currently, no single screening procedure can detect 100% of all breast cancers. Digital Infrared Imaging is designed to be used as an additional procedure with mammography, and other tests, and not as a replacement. Studies show that when DII is added to a woman’s regular breast health check ups (physical examination + mammography + DII), 95% of all early stage cancers may be detected. This would give the vast majority of women who are diagnosed with this disease the reality of returning to a normal healthy life.
 What if we could add another procedure that may act as an early risk marker for this terrible disease? What if we could provide a multi-modal approach that includes technologies that increase the early detection process? Would this give women a better chance for survival? The number of women who die from this disease will change very little if nothing is done to provide a better system. Digital Infrared Imaging has the unique ability to warn some women far enough in advance to give them a fighting chance. Combined with its ability to play a possible role in prevention, the advantages are obvious. With the addition of DII to a woman’s regular breast health care, women of all ages are given an early detection edge in the battle against breast cancer.
What if we could add another procedure that may act as an early risk marker for this terrible disease? What if we could provide a multi-modal approach that includes technologies that increase the early detection process? Would this give women a better chance for survival? The number of women who die from this disease will change very little if nothing is done to provide a better system. Digital Infrared Imaging has the unique ability to warn some women far enough in advance to give them a fighting chance. Combined with its ability to play a possible role in prevention, the advantages are obvious. With the addition of DII to a woman’s regular breast health care, women of all ages are given an early detection edge in the battle against breast cancer.
About the authors:
William Hobbins, MD, a Fellow of the American Board of Surgeons and a board certified clinical thermologist, has been performing thermal breast imaging for over 40 years. As an internationally recognized authority in this field, he has sat on multiple medical and thermographic boards, authored numerous articles, and has contributed a significant amount of research to the medical database using this technology. He currently practices in Madison Wisconsin.
William Amalu, DC, a Fellow of the International Academy of Clinical Thermology and a board certified clinical thermologist, has utilized digital infrared imaging in practice for over 20 years. He is currently the President of the International Academy of Clinical Thermology and the Medical Director of the International Association of Certified Thermographers. Dr. Amalu is in private practice in Redwood City California. For more information, please go to www.breastthermography.com.
https://www.highbeam.com/doc/1G1-196323177.htmlMedical Devices and Systems
The following is Chapter 25 of the 2006 edition of The Biomedical Engineering Handbook, Third Edition, Medical Devices and Systems published by CRC Press. Joseph D. Bronzino, editor of the handbook, comments that “Medical Devices and Systems” is an authoritative reference text and is considered the “bible” of biomedical engineering. This latest volume presents new and updated material contributed by a team of world-renowned experts. The text reflects the most recent advances in both research and practice, and authoritatively covers sensor and imaging technologies, signal analysis, and medical instrumentation. This Third Edition presents an excellent summary of the status of knowledge and activities of biomedical engineers in the beginning of the 21st century.” The principle author of this chapter, Dr. William Amalu, is joined by three other world-renowned experts in this field to present the state-of-the-art in infrared breast imaging. The following chapter contains a review of the literature along with a presentation of infrared physics, imaging system standards, a brief historical background, laboratory and patient imaging standards and protocols, and a look at the future of this lifesaving technology.The following is a brief highlight of the chapter that follows:
• In 1982, the FDA approved breast thermography as an adjunctive breast cancer screening procedure. • Breast thermography has undergone extensive research since the late 1950’s. • Over 30 years of research comprising over 800 peer-reviewed studies on breast thermography exist in the index-medicus literature. • In this database, well over 300,000 women have been included as study participants. • The numbers of participants in many studies are very large — 10K, 37K, 60K, 85K … • Some of these studies have followed patients up to 12 years. • Strict standardized interpretation protocols have been established for over 15 years. • Breast thermography has an average sensitivity and specificity of 90%. • An abnormal thermogram is 10 times more significant as a future risk indicator for breast cancer than a first order family history of the disease. • A persistent abnormal thermogram caries with it a 22x higher risk of future breast cancer. • An abnormal infrared image is the single most important marker of high risk for developing breast cancer. • Breast thermography has the ability to detect the first signs that a cancer may be forming up to 10 years before any other procedure can detect it. • Research has shown that breast thermography significantly augments the long-term survival rates of its recipients by as much as 61%. • When used as part of a multi-modal approach (clinical examination + mammography + thermography) 95% of early stage cancers will be detected. https://books.google.com/books/about/The_Biomedical_Engineering_Handbook_1.html?id=6bK84ZHFuW4CCircadian rhythm chaos: a new breast cancer marker.
Keith LG, Oleszczuk JJ, Laguens M.; Department of Obstetrics and Gynecology, Northwestern University Medical School, Chicago, Illinois, USA. The most disappointing aspect of breast cancer treatment as a public health issue has been the failure of screening to improve mortality figures. Since treatment of latestage cancer has indeed advanced, mortality can only be decreased by improving the rate of early diagnosis. From the mid-1950s to the mid-1970s, it was expected that thermography would hold the key to breast cancer detection, as surface temperature increases overlying malignant tumors had been demonstrated by thermographic imaging. Unfortunately, detection of the 1-3 degrees C thermal differences failed to bear out its promise in early identification of cancer. In the intervening two-and-a-half decades, three new factors have emerged: it is now apparent that breast cancer has a lengthy genesis; a long-established tumor-even one of a certain minimum size-induces increased arterial/capillary vascularity in its vicinity; and thermal variations that characterize tissue metabolism are circadian (“about 24 hours”) in periodicity. This paper reviews the evidence for a connection between disturbances of circadian rhythms and breast cancer. Furthermore, a scheme is proposed in which circadian rhythm “chaos” is taken as a signal of high risk for breast cancer even in the absence of mammographic evidence of neoplasm or a palpable tumor. Recent studies along this line suggest that an abnormal thermal sign, in the light of our present knowledge of breast cancer, is ten times as important an indication as is family history data. https://www.ncbi.nlm.nih.gov/pubmed/11720196Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer.
Ng EY, Sudharsan NM. Source School of Mechanical and Production Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. mykng@ntu.edu.sgABSTRACT
BACKGROUND
Mathematical modeling and analysis is now accepted in the engineering design on par with experimental approaches. Computer simulations enable one to perform several ‘what-if’ analysis cost effectively. High speed computers and low cost of memory has helped in simulating large-scale models in a relatively shorter time frame. The possibility of extending numerical modeling in the area of breast cancer detection in conjunction with medical thermography is considered in this work.METHODS
Thermography enables one to see the temperature pattern and look for abnormality. In a thermogram there is no radiation risk as it only captures the infrared radiation from the skin and is totally painless. But, a thermogram is only a test of physiology, whereas a mammogram is a test of anatomy. It is hoped that a thermogram along with numerical modeling will serve as an adjunct tool. Presently mammogram is the ‘gold-standard’ in breast cancer detection. But the interpretation of a mammogram is largely dependent on the radiologist. Therefore, a thermogram that looks into the physiological changes in combination with numerical simulation performing ‘what-if’ analysis could act as an adjunct tool to mammography.RESULTS
The proposed framework suggested that it could reduce the occurrence of false-negative/positive cases.CONCLUSION
A numerical bioheat model of a female breast is developed and simulated. The results are compared with experimental results. The possibility of this method as an early detection tool is discussed. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC416480/Medical Infrared Imaging of the Breast: An Analysis of 100 Successive Cases of Breast Cancer
William C. Amalu, DC, DABCT, FIACT PCRC Infrared Imaging Lab – Redwood City, California March 18, 2015 The following data presents the findings in 100 successive cases of breast cancer using medical infrared imaging (MIR). Thermovascular markers were detected using a specialized high-resolution computerized medical infrared imaging system capable of detecting minute variations in the regional vascular perfusion of the microdermal circulation. The imaging system used is composed of a highly sensitive infrared camera coupled to a central processing unit capable of multitasking capabilities including post-image processing and accurate temperature measurements (Spectron IR 640 Medical Infrared Imaging System). All pre-imaging patient preparation protocols and laboratory requirements were strictly adhered to as per established MIR standards and guidelines. Following MIR interpretation guidelines, each patient was referred back to their primary care provider with recommendations for follow-up imaging or testing. The final diagnosis in each case was made by biopsy. In the data presented is a special category of patients. In this group MIR was the first alarm that anything was wrong. If it were not for MIR all of these patients would not have known they had breast cancer. When analyzing each breast, 20 basic thermal attributes are used in the grading process. A computerized analysis of both thermovascular patterns and objective temperature values are compared to a normative database. This determines where each breast is graded into one of five thermobiological classifications: TH1 – uniform non-vascular TH2 – uniform vascular TH3 – questionable TH4 – abnormal TH5 – very abnormal The following is a summary of the MIR findings in 100 successive cases of breast cancer – Thermobiological Grade 3 (TH3 – questionable): 22 cases- Of the 22 cases, 10 were “first alarm” thermograms.
- Of the 22 cases, only 9 were true TH3s. The remaining 13 cases were TH3+ (TH3+ thermograms are almost TH4s)
- Of the 22 cases, 2 cases had bilateral breast cancer graded TH3 in both breasts.
- One case was only 28 years old. This was the youngest patient in all of the 100 cases.
- Of the 22 cases, 10 cancers were in the right breast and 12 in the left breast.
- Of the 43 cases, 22 were “first alarm” thermograms.
- Of the 43 cases, 1 case had bilateral breast cancer graded TH3 in one breast and TH4 in the other.
- Of the 43 cases, 1 patient was pregnant.
- Of the 43 cases, 1 patient had a 3 year lead-time thermogram warning.
- Of the 43 cases, 14 cancers were in the right breast and 29 in the left breast.
- Of the 35 cases, 18 were “first alarm” thermograms.
- One case was only 36 years old.
- Of the 35 cases: 3 were TH6s, 2 were TH7s, 2 were TH8s, and 1 case was a TH9
- Of the 35 cases, 1 patient had a 4 year lead-time thermogram warning.
- Of the 43 cases, 13 cancers were in the right breast and 22 in the left breast.