Medical Infrared Imaging Products

CAMERA- Spectron IR 640×480**
- TyTron C-500 IR
- Standard 50mm camera lens
- Detector: 640 x 480 VOx Focal Plane Array
- True 640×480 resolution providing 307,200 pixels
- Spectral Band: 8-12 Microns
- Frame Rate: 30 Hz
- Spatial Resolution: 0.50 mrad
- Sensitivity: less than 50 mK average
- Computer Interface: Digital (Ethernet)
- Focusing Distance: 12 inches to 15 feet
- Software Controlled Electronic Focus
- Heavy duty machined aluminum camera body for superior thermal stability
- Narrow temperature range specifically designed for human tissue imaging
- Front shutter design to increase accuracy and stability, while reducing the need for factory calibration
- Master calibration feature that calibrates the camera to your room temp which increases accuracy and stability, while reducing the need for factory calibration
- 1 year warranty
- Accurate on-screen temperature differentiation (Delta-T)

Spectron IR Basic System
- Dedicated medical 640×480 resolution infrared camera
- Spectron IR professional medical software
- Professional, full analytical software features
- Dual pole design provides independent IR camera and computer movement
- Height adjustable work surface for seated or standing operation
- Collapsible stand featuring simple, quick and easy setup
- Pivoting camera arm
- Manual squeeze clamps accommodate image acquisition
- Dedicated and integrated laptop
- Ideal for all offices including mobile
- Sturdy base
- 1 year warranty
- 1 year free technical support included
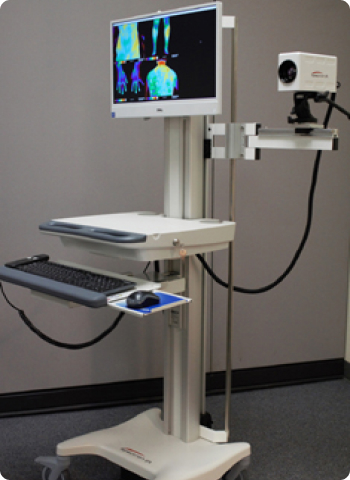
Spectron IR Medical Imaging Workstation System
- Dedicated medical 640×480 resolution infrared camera
- Spectron IR professional medical software
- Professional, full analytical software features
- Spectron IR U.S. patented Imaging Workstation
- Medical grade, fully self contained system
- Large 4 wheel base with locking castors
- Simple, automated, remote controlled camera height adjustment decreases imaging times by nearly 50%
- Height adjustable work surface for seated or standing operation
- Pivoting camera arm
- Pan and tilt camera arm
- Dedicated and integrated computer
- Streamlined imaging process
- 1 year warranty
- 1 year free technical support included
- Designed for ease-of-use in hospital or clinical environment

All-In-One Computer. CPU
- Integrated computer with software installed
- Windows operating system
- WiFi enabled
- Wireless mouse
- Wireless keyboard
- CAT 6 Ethernet Cable
FDA Indications for Use 510(k) K032471: Intended for adjunctive diagnostic screening for the detection of breast cancer and other uses
such as: peripheral vascular disease, neuromusculoskeletal disorders, extracranial cerebral facial vascular disease, thyroid gland
abnormalities, and various other neoplastic,metabolic, and inflammatory conditions.
Use of the TyTron C-500 is not intended to be a sole diagnostic procedure for these diseases and conditions.
*MOBILE PORTABLE UNITS AND ENTRY LEVEL SOLUTIONS AVAILABLE CLICK HERE TO VIEW CONFIGURATIONS*